

Summary. Allelic relationships of the multinodosum character of barley in two different mutant lines, one depicting multinodosum condition at early stage and other at later stage was investigated.
The early stage multinodosum mutant of variety 'C 164' was found to be non-allelic to the late stage multinodosum mutant of variety 'HBL 98'. A good fit for 9 normal: 3 late stage multinodosum: 4 early stage multinodosum ratio for F2 segregants indicated the control of the character under two genes displaying recessive gene interaction. The F2 study also revealed that multinodosum mutants had arisen from the normal by recessive mutation for one gene at either locus (Mn1 Mn1 Mn2 Mn2 -> Mn1 Mn1 mn2 mn2 or mn1 mn1 Mn2 Mn2) one depicting the trait at an earlier stage and another at a later stage. The double-recessive multinodosum recombinants observed in F2 generation (with larger number of nodes than in mutant parents) were considered to have the cumulative effect for number of nodes. It was, however, not possible to confirm the results obtained during the F2 generation through the F3 study in which the F2 multinodosum plants with number of nodes greater than mutant parents segregated for normal and multinodosum plants against expectation, calling for further clarification for the inheritance of this trait.
Introduction. Barley varieties generally have number of nodes three to five in their main stem. Multinodosum mutants of barley are characterized by presence of nodes greater than five in the main stem.
In previous studies, Minocha and Brar (1969) through the M3 generation study, Gill and Sethi (1970) through morphogenetics of multinodosum and Shvedov (1981) from his allelism work on multinodosum mutants found that the character was controlled by a single recessive gene.
This paper presents the results obtained in the study of two different multinodosum mutants. The objectives of the study were: (a) to ascertain the number of genes controlling expression of the mutant phenotype (b) to ascertain the allelic relationships of the genes and (c) to study cumulative effect of non-allelic genes.
Materials and Methods. The two multinodosum mutants involved in the study and the source of each are given in Table 1. The mutants were obtained from the Department of Plant Breeding, Himachal Pradesh Kirshi Vishva Vidyalaya, Palampurp India by EMS (Ethyl methane sulphonate) treatments. The line designated 'mt1' was a mutant of variety 'C 164' (average number of nodes = 4.00) and had average number of nodes 9.80. The multinodosum expression in this mutant was at early stage of plant development i.e., before spike emergence. The line designated 'mt2' was a mutant of variety 'HBL 98' (average number of nodes = 4.00) and had average number of nodes 6.80. The multinodosum expression in this mutant was at late stage of plant development i.e., at the spike emergence.
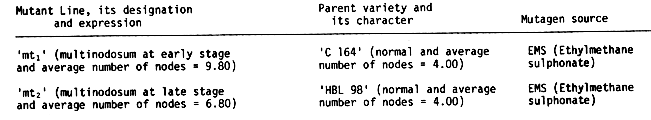
Crosses between these mutant lines were made and information regarding allelic relationships of genes for the character was obtained from F1 phenotypic study and F2 segregation pattern and for ascertaining number of genes the observed counts were compared with expected in F2 generation. Breeding behaviour of the expected F2 genotypes was confirmed in F3 generation.
For the sake of convenience the already assigned gene symbol Mn/mn was used for normal vs. multinodosum. The number assigned to the gene symbol are tentative for the want of their allelic relationship with the known marker.
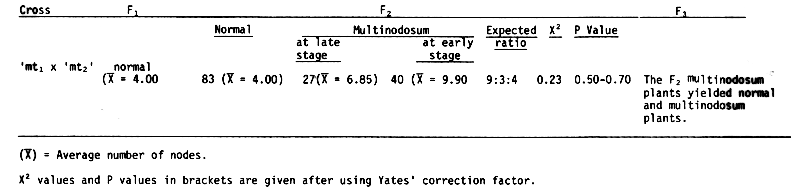
Results and Discussion. The F1 population of the cross between the mutant lines 'mt1' (multinodosum at early stage and average number of nodes = 9.80) and 'mt2' (multinodosum at late stage and average number of nodes = 6.80), had normal number of nodes (average number of nodes above the crown = 4.00) indicating that the two mutants were non-allelic i.e., they complemented each other for wild type. The F1 study also revealed the dominance of normal number of nodes over multinodosum (Table 2).
The F2 population consisting of 83 with normal number of nodes, 27 with late stage multinodosum and 40 with early stage multinodosum condition gave a good fit for 9:3:4 ratio (X2 = 0.23, P = 0.50 - 0.70) suggested a two factor control of the character, exhibiting recessive epistasis. The genotypes and phenotypes of the mutants and their F1 and F2 populations are presented in Figure 1. The F2 study also led to conclude that multinodosum trait had arisen by recessive mutation at either locus (Mn1 Mn1 Mn2 Mn2 -> Mn1 Mn1 mn2 mn2 or mn1 mn1 Mn2 Mn2), one depicting the trait at an earlier stage (as in 'mt1') and other at a later stage (as in 'mt2'). This interpretation has been made keeping in view that there is variety of occurrence of two simultaneous mutations at different loci for the same trait. Further evidence is required to justify similar hypotheses for other characters. This situation appears to be a not uncommon situation in barley, when it is considered that such characters as blue aleurone (Smith, 1951), long weak basal internode (Kasha and Walker, 1961) and possibly accordion rachis (Enns, 1961) are under this form of control.
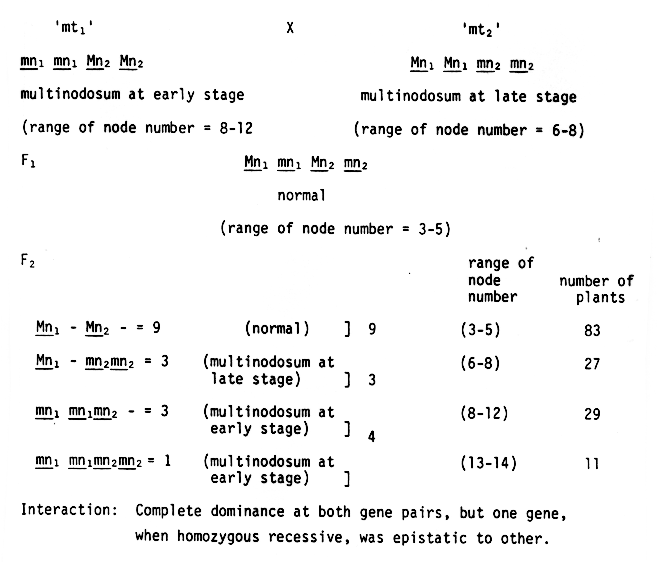
The recombinants showing the multinodosum trait expression at early stage and the number of nodes markedly higher than in either of the mutant parents were considered to be double-recessive (mn1 mn1 mn2 mn2) multinodosum plants. The F3 study, however, failed to confirm the results obtained during the F2 generation, since the multinodosum plants progenies during this generation segregated for normal and multinodosum plants as against expectations (only multinodosum plants progenies were expected from the multinodosum F2 plants). This indicated the presence of either incomplete penetrance and variable expressivity for the multiodosum genes or some other more complex inheritance pattern of the character and therefore, needs further clarification.
References:
Enns, H. 1961. Inheritance and linkage studies in barley using chromosomal interchange and marker stocks. Ph.D. Thesis, Univ. Saskatchewan.
Gill, K. S. and G. S. Smith. 1970. The morphogenetics of induced multinodosum with monopodial branching in barley. Euphytica 19:390-393.
Kasha, K. and w. R. Walker. 1961. Several secent barley mutants and their linkages. Can. J. Genet. Cytol. 2:397-415.
Minocha, J. L. and D. S. Brar. 1969. Many-noded and nodal branched mutants in barley. Indian J. Genet. 29:446-447.
Shvedov, G. G. 1981. Inheritance and allelism of many-noded barley mutants. Referativnyi Zhurna 1065:123.
Smith, L. 1951. Cytology and genetics of barley. Botan. Rev. 17:1-355.