

The inheritance of resistance to barley stripe mosaic virus (BSMV) has been reported in several papers (Sisler and Timian, 1956; Vasquez et al., 1974; Carroll et al., 1979). Sisler and Timian (1956) reported that two recessive genes controlled reaction of Modjo (CI 3212) to the California "E" isolate of BSMV. They stated that reaction to BSMV was not associated with N, hull attachment; B, black lemma; K, hooded lemma; or S, rachilla hair Tength loci. Nilan (1964) suggested the gene symbols sm and sm2 for these BSMV resistance genes. Vasquez et al. (1974) proposed a multiple allelic series controlled reaction to CV18 (Type strain) and that another recessive gene conditioned reaction to strain ND 1. They reported that the loci (sm3 and sm5) controlling reaction to CV18 was inherited independently from genes for stem rust reaction, T; aleurone color, B1; spike row number, V; and rachilla hair length, S. Carroll et al. (1979) reported that a single recessive gene conditioned resistance to seed transmission of the M1-3 isolate of BSMV in Modjo.
The F2 progeny from a diallel cross among the barley cultivars Moreval (CI 5724), Modjo 1 (CI 14048), and CI 4197 were tested for reaction to infection with strain CV42 (California Moderate Oat Strain) of BSMV. All the F2 plants tested were immune to CV42 (Timian, personal communication). This demonstrated that the three cultivars have the same gene for resistance to the CV42 strain of BSMV. Moreval and CI 4197 were selected as geographically different sources of BSMV resistance in an attempt to place the gene(s) for BSMV resistance into Black Hulless (CI 666). Previously, Black Hulless was shown to be very suscep- tible to all strains of BSMV tested. A male sterile gene, msg, was backcrossed into Black Hulless by R. L. Kiesling, Department of Plant Pathology, North Dakota State University, to facilitate crosses to the recurrent parent. A male sterile BC5F3 plant was crossed to Moreval and CI 4197. The F1 plants were crossed to male sterile Black Hulless and CV42 resistant F3 derived lines from the BC1 generation were isolated based on seedling reaction following repeated inoculations with CV42 and a subsequent ELISA serological test for BSMV. To examine linkage relationships the CV42 resistant F3 lines from the Black Hulless *2/Moreval and Black Hulless *2/CI 4197 crosses were crossed as female parents to R. I. Wolfe's Multiple Recessive Master Stock (MR). The hybrids were heterozygous for genes at the wx, n, lk2, v, re2, al, rb, o, s, and r loci (Franckowiak, 1986). Backcross one F1 plants were from the cross Blck Hulless *2/CI 4197//2* MR were scored for the 1 marker genes. Fifty seeds from each F1 plant were planted in pots in the greenhouse and the seedlings were inoculated three times at 14 day intervals. Before the second and third inoculation and after the third inoculation, infected seedlings were pulled. The number of susceptible seedlings were recorded as a percentage of the total seedlings. For summarization of the data, progenies with 10% or more surviving seedlings were considered as segregating for BSMV reaction. Tabulation of the BSMV and marker gene data for individual F2 derived progenies showed an association-between the awn length, Lk2, locus located on the short arm of chromosome 1 and BSMV reaction (Table 1).
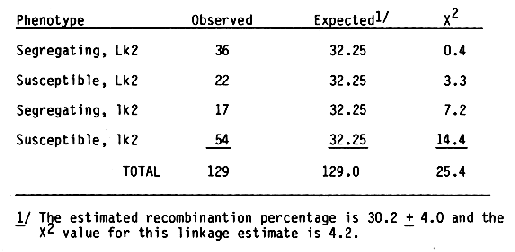
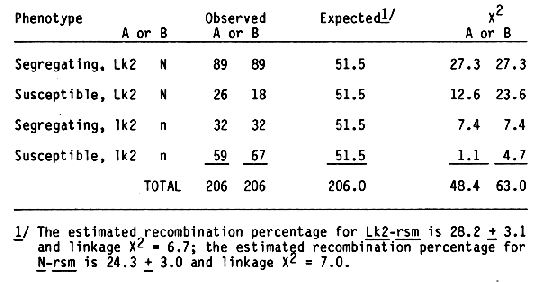
To reexamine the apparent linkage between the Lk2 locus and the locus for reactions to the CV42 strain of BSMV, F1 plants from the cross Black Hulless *2/Moreval//2* MR were scored for marker genes. Fifty seeds from each F1 plan were planted and inoculated twice as seedlings with the CV42 strain of BSMV. The susceptible plants were pulled two weeks after each inoculation. The frequency of surviving seedlings were higher than expected; therefore, F2 progenies with less than 30% surviving seedlings were considered susceptible. Summarization of the data (Table 2) showed more segregating F2 progenies than expected. Since both recombinant classes, long awn-susceptible and short awn-segregating, were deficient, the results indicated that the Lk2 and BSMV response loci are linked. Since the female parent in this cross had covered seed, linkage data for the N. hull attachment, and RSMV response was obtained. As expected, both loci were linked (Table 2).
Recombinant percentage estimates for the Lk2 and BSMV response loci for the two crosses were 30.2 ± 4.0 and 28.2 ± 3.1, respectively. As expected, the BSMV response locus was linked to the N locus; estimated recombination value is 24.3 ± 3.0. Because the frequency of surviving seedlings in the Black Hulless *2/Moreval//2* MR cross was high, the linear sequence for the three loci can not be determined. Previously, Sisler and Timian (1956) reported that BSMV response and the N locus were not linked. Different BSMV isolates were used in the .studies; hence, a new gene symbol, rsm, is suggested for the BSMV response isolated in this study. In another study, 1000 F2 plants from the cross Black Hulless *2/CI 4197//MR were inoculated repeatedly with the CV42 strain of BSMV. One hundred ninety-six individual seedlings showing no symptoms and reacting negatively for BSMV in ELISA serological tests were transplanted to six-inch pots. After data on morphological markers of individual plants were accumulated, 177 of the plants were found to have long awns and 19 had short awns. The chi-square value for a 3:1 expected ratio is 24.5; thus, one factor for BSMV response appeared to be linked with the Lk2 locus. Subsequent inoculation of seedlings from the surviving plants with CV42 demonstrated that the rsm factor for BSMV response was not the only factor required for resistance to the CV42 strain of BSMV (Table 3). None of the 50 progenies examined contained only resistance or susceptible seedlings; thus, segregation for another major factor affecting BSMV response is unlikely.
Table 3. The reaction of F3 seedlings derived from the BSMV resistant F2 plants from the cross Black Hulless *2/CI 4197//MR to the CV42 strain of BSMV. Acknowledgement: Thanks are due Mr. Kerman Alm for technical assistance in this research.
References:
Carroll, T. W., p. L. Gossel, and E. A. Hockett. 1979. Inheritance of resistance to seed transmission of barley stripe mosaic virus in barley. Phytopathology 69:431-433.
Franckowiak, J. D. 1986. Multiple marker stocks from Beaverlodge, Canada. Barley Genet. Newsl.16:125-126.
Nilan, R. A. 1964. The cytology and genetics of barley 1951-1962. Monogrpahic Suppl. 3, Res. Stud. Vol. 32, No. 1, Washington State University, Pullman.
Sisler, W. W. and R. G. Timian. 1956. Inheritance of the barley stripe mosaic resistance of Modjo (C.I. 3212) and C.I. 3212-1. Plant Dis. Rep. 40:1106-1107.
Vasquez, G. G., G. A. Peterson, and R. G. Timian. 1974. Inheritance of barley stripe mosaic reactin in crosses among three barley varieties. Crop Sci. 14:429-432.