Barley Genetics Newsletter 37: 34–36 (2007)
Four new barley mutants
Sandra AI Wright1, Manochehr Azarang2, Anders B Falk3
1Dipartimento di Scienze Animali, Vegetali e dell’Ambiente, Facoltà di Agraria, Università del Molise, Campobasso, Italy
2Maselaboratorierna, Uppsala, Sweden 3Department of Plant Biology and Forest Genetics, University of Agricultural Sciences, Uppsala, Sweden
Introduction
We screened a fast-neutron mutated barley population to isolate and characterize barley lesion mimic mutants. The albino mutant frequency in this population was found to be around 2%, suggesting that a sufficiently high level of mutants could potentially be found. From a screen of about 5000 M2 spikes, we found four mutants that were characterized by the presence of necrotic or chlorotic leaf areas in the form of spots or stripes. The four mutants were designated 1661, 2721, 3091 and 3550. The mutants were characterized with respect to their visual appearance. In order to relate these mutants properly to previously described barley lesion mimic mutants, we cultivated all previously described barley mutants with aberrant leaf phenotypes (Davis et al.. 1997) and compared the phenotypes to those of the newly obtained mutants.
Materials and Methods
The near-isogenic barley line Bowman(Rph3) was obtained from Dr Jerry D. Franckowiak, North Dakota, USA. It was constructed by introgression of the Rph3 resistance gene from the cultivar Estate into the cultivar Bowman, and represents the 7th backcross. Mutation was performed with fast neutrons at the International Atomic Energy Agency (IAEA), Vienna, Austria, with a dose of 5 Gy. Five thousand M2 spikes were sown and the mutant plants were screened for aberrant leaf phenotype. Selected mutants were backcrossed twice to wildtype Bowman (Rph3), and their phenotypes were analyzed using the backcrossed material. Plants were grown either in a greenhouse or in caged outdoor compartments during the summer. Experiments were done in controlled growth chambers at 22°C with 16/8 hours of light/darkness (long day conditions), or 8/16 hours of light/darkness (short day conditions). Allelism tests were done as inspections of leaf phenotypes of F1 plants resulting from crosses between relevant mutants. An AFLP-based procedure was used to screen for molecular markers linked to the mutants (Castiglioni et al. 1998).
Results and Discussion
Mutant 1661 displays chlorotic stripes (Figure 1). These stripes are most pronounced on the first leaf. Under long day conditions, the stripes do not appear on the later emerging leaves and the plants eventually seem to recover from the phenotype conferred by the mutation. Under short day conditions the mutation is semi-lethal since all leaves develop the characteristic chlorotic stripes and the plants fail to reach maturity and produce seeds. However, the phenotype initially proves to be more severe under long day conditions. Mutant 1661 is phenotypically similar to the previously described mutants mottled leaf 1 and mottled leaf 5, which have clearly marked white bands across the leaves (Davis et al. 1997). Allelism tests indicated that 1661 is not allelic to these. None of the pre-mapped Proctor-Nudinka AFLP markers were linked to mutant 1661.
Mutant 2721 is characterized by chlorotic leaf spots and streaks that coalesce and eventually form large white patches on the leaves (Figure 1). The phenotype is displayed on all leaves and as the leaves mature, the white regions gradually become darker, seeming to undergo necrosis and death. Often the necrosis affects the leaf edges, resulting in wrinkled leaf edges. Under short day conditions, the leaf phenotype is delayed by several days and is reduced in severity. Eventually all leaves display the phenotype even under short day conditions. The mutant initially appears to be similar to the mottled leaf 2 (Davis et al. 1997) and mottled leaf 6 (Franckowiak 2002) mutants, which display yellow bands on the leaves. However, these mutants do not display the necrosis of 2721. Allelism tests suggest that 2721 is not allelic to the mottled leaf mutants. None of the pre-mapped Proctor-Nudinka AFLP markers were linked to mutant 2721.
Mutant 3091 has brown spots towards the leaf tips and leaf edges, particularly on the first leaf (Figure 1). Short days do not significantly alter the phenotype. The leaf phenotype resembles those of mutants nec4 and nec5 (Davis et al.. 1997). However, allelism tests indicate that mutant 3091 is not allelic to either of these two. For the mutation in 3091, linkage was detected to the AFLP marker E37M33-6 on barley chromosome 3 (3H).
Mutant 3550 has conspicuous black or brown spots on the leaves (Figure 1). The spots usually do not coalesce. They appear on all above-ground parts of the plant including the bristles. Mutant 3550 is delayed in maturation and ripening, with seeds being ready for harvest about four weeks later than in the wildtype. Short day conditions lead to a slightly less pronounced phenotype. Mutant 3550 is similar to the nec1 mutant (Davis et al. 1997), but allelism could be ruled out due to different mapping positions. The mutation in 3550 was localized on chromosome 7 (5H). Linkage was detected to the AFLP markers E40M38-7, E36M36-5, E42M36-14, E42M40-2 and E41M32-5.
References:
Castiglioni, P., Pozzi, C., Heun, M., Terzi, V., Müller, K.J., Rodhe, W. and Salamini, F. 1998. An AFLP-based procedure for the efficient mapping of mutations and DNA probes in barley. Genetics 149:2039–2056.
Davis, M.P., J.D. Franckowiak, T. Konishi, and U. Lundqvist, 1997. New and revised descriptions of barley genes. Barley Genetics Newsletter 26: 22-516.
Franckowiak, J.D. 2002., BGS 629 Mottled leaf 6. Barley Genetics Newsletter 32:170.
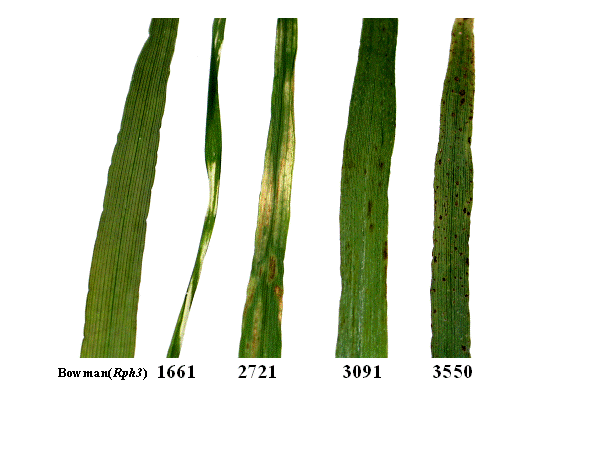
Figure 1. Leaf phenotypes of the parent cultivar Bowman(Rph3) and four lesion mimic mutants; 1661, 2721, 3091, 3550.